Abstract
GATA2 Zinc-Finger (ZF) mutations are associated with specific subgroups of myeloid malignancies. Alterations of the N-terminal ZF1 were identified in AML patients with biallelic CEBPA mutations, whereas the C-terminal ZF2 is typically affected by germline mutations, predisposing to MDS and AML, or by somatic lesions in CML blast crisis. Nevertheless, the context-dependent mechanism underlying GATA2 ZF2 mutations remains mostly unclear. Here, we set out to study the functional consequences of GATA2 ZF mutations in myeloid malignancies.
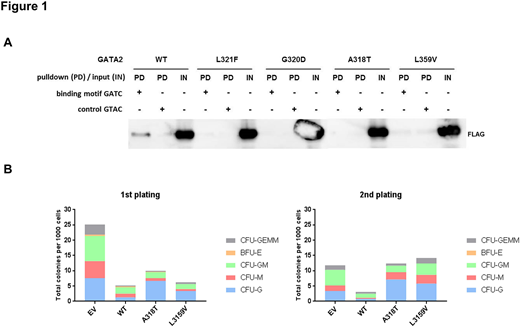
In particular, we performed DNA-pulldown experiments, using FLAG-tagged full length GATA2 ZF WT and mutant proteins after expression in HEK293T cells and incubation of the cell lysates with biotinylated oligonucleotides encoding the binding-motif GATC. These experiments showed disruption of DNA binding for all GATA2 ZF mutants tested in vitro - regardless of the mutant positions within the ZF domains (Figure 1 A).
Moreover, we studied the impact of GATA2 ZF mutations on the protein-interaction with Friend of GATA Protein 1 (FOG1; HGNC symbol ZFPM1). The influence of FOG1 on GATA2-dependent transcriptional activation was evaluated using a GATA-specific luciferase reporter. All GATA2 mutants tested were able to activate this reporter, although to variable extent. While co-expression of FOG1 overall counteracted GATA2-dependent transcriptional activation, this effect was significantly reduced for the GATA2 mutants L321F (located in ZF1) and T354M (located in ZF2). This suggested that both ZF domains are involved in the FOG1-interaction.
To gain insights into the influence of GATA2 ZF1 mutation on hematopoiesis we performed colony forming cell (CFC)-assays. Lin- primary murine bone marrow cells expressing GATA2 WT or mutants were plated in methylcellulose supplemented with cytokines. Consistent with previous reports, GATA2 WT expression led to a reduced colony number, while this effect was decreased for both mutants A318T (ZF1) and L359V (ZF2). In particular, a higher number of CFU-G colonies were observed for the GATA2 mutant-expressing cells indicating a lineage shift towards granulopoiesis (Figure 1 B).
In summary, we have shown that GATA2 mutations influence DNA-binding, protein-interactions and myeloid differentiation. Our findings further suggest that GATA2 ZF1 mutations may contribute to myeloid leukemogenesis through increased proliferation of granulocytic progenitors. Understanding the oncogenic collaboration of GATA2 mutations with other driver genes in distinct patient subgroups is a challenge ahead.
Hiddemann:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal